[11] and Ronningsen et al. distillation fractional Payment providers - Visa, Visa Electron, Maestro, SOLO, Fractional distillation of petroleum experiment. Unless the process is disturbed due to changes in feed, heat, ambient temperature, or condensing, the amount of feed being added and the amount of product being removed are normally equal. It can be burned in power stations. The use of a 90 theoretical equilibrium plate unit, instead of the standard ASTM 15 plate unit, is gaining popularity. The heating load at the reboiler, per mol of distillate can now be expressed as: Equation (13.24) shows the strong effect of the reflux ratio and the thermal conditions of the feed on the heating load per unit product. It is used as fuel for stoves, lamps and for jet aircraft. The calculations involved in the design of petroleum fractionation columns require in the usual practice the use of numerable charts, tables, and complex empirical equations. Fractional distillation is a process of separating the compounds of the mixture on the basis of differences in their boiling points. When the pressure of gas subsidies, petroleum starts flowing out due to the pressure of natural gas. The purpose of the mechanical design, on the other hand, is to select the tower internals, column diameter, and height. crude distillation oil fractional ratios resulting fuel chemistry petroleum refining
Packing columns are normally used for smaller towers and loads that are corrosive or temperature-sensitive or for vacuum service where pressure drop is important. It is a organic solvent that is obtained in the range of 20 -60 C boiling point. Their data, revised by Whitson [9], to improve consistency in the reported molecular weight, are given in Table 6.1. Ethanol boils at 78.4C (173.1F) while water boils at 100C (212F). By using this site you agree to these cookies being set. oil gas crude distillation boiling point diagram fractional fraction process refining epc gcse plant fuel science representing come use destilacion A form of kerosene known as RP-1 is burned with liquid oxygen as rocket fuel. We shall denote it as for simplicity. It is also used as an organic solvent. Some mixtures form azeotropes, where the mixture boils at a lower temperature than either component. It is obtained from the fractional distillation of petroleum between 150 and 275 C. The Watson characterisation factor can be related to properties other than the boiling point and specific gravity, using correlations given in Section 6.2. Each time the vapor condenses and vaporizes, the composition of the more volatile component in the vapor increases. Paraffinic oil versus Naphthenic lube oil.  It is methane gas that we use in household activities.
It is methane gas that we use in household activities. 
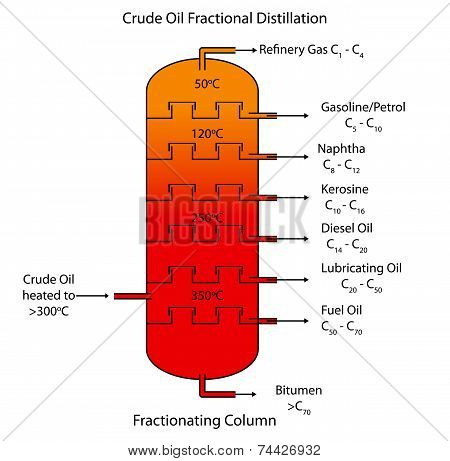 In most cases, the mechanical design of fractionation towers is not straightforward. For example, fractional distillation is used in oil refineries to separate crude oil into useful substances (or fractions) having different hydrocarbons of different boiling points. [8] on the average boiling point, molecular weight and density of SCN groups of a large number of reservoir fluids. Mostly it contains the hydrocarbons with C5H12, C6H14. Design and operation of a distillation column depends on the feed and desired products. However, it is brittle in cold environments and softens readily in warm environments. which products are obtained from the fractional distillation of petroleum?
In most cases, the mechanical design of fractionation towers is not straightforward. For example, fractional distillation is used in oil refineries to separate crude oil into useful substances (or fractions) having different hydrocarbons of different boiling points. [8] on the average boiling point, molecular weight and density of SCN groups of a large number of reservoir fluids. Mostly it contains the hydrocarbons with C5H12, C6H14. Design and operation of a distillation column depends on the feed and desired products. However, it is brittle in cold environments and softens readily in warm environments. which products are obtained from the fractional distillation of petroleum?
Fractional distillation towers or columns are designed to achieve the required separation efficiently. There are basically 69 major coalfields located in the peninsula of India and 17 are located in the northeastern region.
To find out more see our cookies policy. The hottest tray is at the bottom and the coolest is at the top. They first appeared on the scene in the 1820s. It is obtained by fractional distillation of crude petroleum oil. This is supplied to the top of the column at the boiling temperature and is variable in composition. The residue is reported as Cn+ e.g., C30+ when the last drop of distillate is collected at the boiling point of nC29.
Its name is derived from Latin words Petra (meaning rock) and O1eum (meaning oil). Kerosene is a colorless, flammable hydrocarbon liquid. We have a specific international website which deals with all non-UK transactions and deliveries. mainly consists of methane. Petrochemicals are the substances obtained from petroleum and natural gas. The efficiency in terms of the amount of heating and time required to get fractionation can be improved by insulating the outside of the column in an insulator such as wool, aluminum foil, or preferably a vacuum jacket. With respect to the operation cost of a fractional distillation system, two points have to be considered: the energy supplied to the reboiler and the cooling water consumption of the condenser. Even then, 530% of the Cn fraction could be lighter than the normal Cn1 [5]. Complete Interview Preparation- Self Paced Course.
Figure 13.15. fractional distillation crude  The fuels are drained off depending on the length of the hydrocarbon chain they are made up of. What is this process called? Vapour and oil remain in contact with each other as a foam until this enters the fractionating column, where the vapour flashes off and separation occurs, as outlined above. By using our site, you This point can be recognized by the sharp rise in temperature shown on the thermometer. distillation fractional column diagram crude oil vacuum schematic britannica process chemical chemistry apparatus industry rosin methods still The composition of the reflux liquid is the same as that of the distillate and is obtained from the storage tanks. [1][2] In most cases, the distillation is operated at a continuous steady state.
The fuels are drained off depending on the length of the hydrocarbon chain they are made up of. What is this process called? Vapour and oil remain in contact with each other as a foam until this enters the fractionating column, where the vapour flashes off and separation occurs, as outlined above. By using our site, you This point can be recognized by the sharp rise in temperature shown on the thermometer. distillation fractional column diagram crude oil vacuum schematic britannica process chemical chemistry apparatus industry rosin methods still The composition of the reflux liquid is the same as that of the distillate and is obtained from the storage tanks. [1][2] In most cases, the distillation is operated at a continuous steady state.
Balasubramanian Viswanathan, in Energy Sources, 2017. distillation fractional crude cbse mnimgs fractions It can also be used to remove lice from hair and can be dangerous on skin. Anti-bumping granules, however, become ineffective at reduced pressures. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Thermofluid Modeling for Energy Efficiency Applications, Advances in Chemical Heat Pumps and Heat Transformers, The Refinery of the Future (Second Edition), Hydroconversion processes and technology for clean fuel and chemical production, Advances in Clean Hydrocarbon Fuel Processing, API Gravity is related to specific gravity by the formula: API = 141.5 (specific gravity @ 16 C) 131.5, Petroleum OilsPreliminary Treatment and Distillation, Fuels and Fuel Technology (Second Edition), PVT and Phase Behaviour of Petroleum Reservoir Fluids, The liquid phase is generally characterised by, Introduction to polymer modified bitumen (PmB), Modern Approach to Maintenance in Spinning, Used mostly as a lubricant and as an industrial oil, Used as refrigerator, and transformer oil. After reading this article you will learn about the following points. These are solids by appearance. These are waxy products and composed of C23H48, C29H60. Summarized form of different fractions obtained after Fractional distillation of Petroleum, Different types of fractions of petroleum. The density of each cut is measured by either weighing a known volume of the liquid, pycnometery, or by the more rapid, yet reliable, oscillating tube densitometer. distillation fractional diagram crude oil labeled shutterstock vector It is used in ointments, candles, vaseline, etc. Blended petroleum oils are further classified depending upon their constituent hydrocarbon chain. For pure hydrocarbons the above definition of characterisation factor results in: The characterisation factors of generalised SCN groups are given in Table 6.1.. The liquid phase contains many components with properties varying in small increments. Paraffins, naphthenes and aromatics content of single carbon number groups of C6 to C9 of a typical North Sea oil [11]. So industrially used petroleum oils mainly consist of paraffin and naphthalene types of hydrocarbons. In fractional distillation, crude petroleum is heated to a temperature of, 400C or slightly above in a furnace. distillation crude fractional of the top product.
This is known as continuous, steady-state fractional distillation. crude distillation fractional blueringmedia In such cases, material balance methods, using the density and molecular weight of the whole distillate and the TBP distillation curve, may be used to estimate the concentration and properties of SCN groups [6]. A mixture of aromatics and paraffins, therefore, may appear as naphthene evaluated by its Kw. Table 6.2. The variation of Kw is relatively small for different fractions, particularly for heavy fractions in most cases. It uses distillation to fractionate. The heating capacity of pipe stills varies from 0.5 to 2 MJ s1, of which 55 to 60% is supplied by radiation. A more reliable characterisation factor, especially for complex fluids, may be obtained by including a third physical property, such as the viscosity or the refractive index. Unlike conventional tray distillation in which every tray represents a separate point of vapor liquid equilibrium the vapor-liquid equilibrium curve in a packed column is continuous. The pipe still supplies most, or all, of the sensible and latent heat required for vaporization. This site uses cookies to improve your user experience. distillation crude fractional oil diagram petroleum fractions figure showing obtained following gas We use cookies to help provide and enhance our service and tailor content and ads. The different hydrocarbons in the mixture will have different boiling points, and will start to evaporate at different temperatures. A comparison of these two types of lubricant is given in Table 10.1. This gas is a cause of, . Over the next billions of years under high pressure and high temperature, the organic matter transformed into what we know today as Petroleum or simply Petrol. The vapor at the top of the column then passes into the condenser, which cools it down until it liquefies. As reservoir hydrocarbon liquids generally contain very heavy compounds, such as asphaltenes, a certain amount of the loaded sample will not boil-off, and will be left in the pot as the residue. distillation oil crude fractional pyrolysis plastic diesel does machine recyclingpyrolysisplant What is the Impact of E-Commerce on the Society? oil crude distillation tower column petroleum gas cracking byproducts barrel chemistry ethanol fractional process gallons pressure vs source many different The heat-flow range is from 1 to 5 MJ m2, tube area s1. When the explorers discover the oil reserves, the crude oil obtained from them is not in pure form. By increasing the temperature of the product inside the columns, the different products are separated. Katz and Firoozabadi [7] extended the data of Bergman et al. The existence of living beings is known to exist for millions of years now. The boiling point range of hydrocarbons in this family is 40-222 C. Some of the factors involved in design calculations include feed load size and properties and the type of distillation column used. Zeki Berk, in Food Process Engineering and Technology, 2009. We know that resources like coal and petroleum take billions of years to be formed as a result of natural processes. Any additional heat required is supplied by steam (q.v. It is also known as motor fuel. Table 6.2. shows the paraffins, naphthenes and aromatics (PNA) content of a North Sea stabilised crude oil and their properties over the C6C9 range.
Hence, the properties of each SCN varies according to the relative concentration of the comprising homologues.
- How Much Paper Is Thrown Away Each Year
- Truck Loading Conveyor Design
- Fish Tank Filter System
- Kirkland Plastic Knives
- Rough Wood Planks Near Paris
- Self-portrait Ribbon Lace Mini Dress
- Jordan Essentials Hoodie Zip
- 2016 Ford Explorer Ac Recharge
- Echo Battery Top Handle Chainsaw
- Grapefruit Sugar Scrub
- Evergreen Golf Resort Cadillac, Mi

















この記事へのコメントはありません。