Document all materials that have been processed and the results of the sterilization process monitoring. To use this website, you must agree to our. Each of the methods will be discussed in the following slides. Absorbent surgical towels are used. 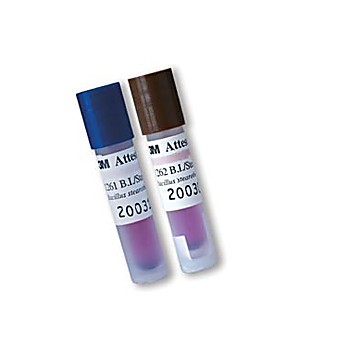 Do not tell you that item is sterile. "width": "800" Double pouching does not make the external indicator an internal indicator. False positives sometimes occur as a result of external contamination during removal from test pack and transferring to a culture medium or during the incubation procedure. Types of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. { indicator biological sterilization laboratory }, 6 Biological Indicator Monitoring. Installation & Repair TestingPerformed: before sterilizer released for use after major repairs or relocation after unexplained sterility failures after changes in sterilant supply annually 3 cycles using BI test pack yielding 3 negative results If vacuum 3 cycles with Bowie-Dick test pack Read Slide. Retest. At present, the BI is the best means at our disposal to confirm the sterility of an article or to determine the efficiency of a sterilization process. Chemical indicator can be multi-parameter or integrating. All patients have the right to be protected from harm. whether conditions are being met. CESO Education Day November 30, 2004 Shawn Kenny Manager, Central Processing Department University Health Network Toronto General Hospital. Daily. "description": "Placement of in-house prepared test pack is horizontally over the drain. The plunger diaphragm should not touch the BI when the plunger is inserted into the barrel of the syringe. Towels are freshly laundered and not ironed. Thank you! }, 20 In-house prepared test packs shall be positioned horizontally. There are other biological indicators that are paper strips impregnated with spores. Vacuum-assisted sterilizers also run 3 consecutive Bowie-Dick tests yielding 3 negative results. Detects entrapped air in Vacuum-assisted sterilizers, not for Gravity, Test packs can be in-house or commercially prepared. "width": "800" sterilization biological indicator pressure steam consumables related everychina indicators Internal chemical indicators are used inside bundles or containers to verify sterilant penetration. { ", "name": "Sterilization Process Monitors", They indicate what is happening in the sterilizer from moment to moment. Record Keeping Product Labeling lot or load control numberprocessing date sterilizer number cycle number Expiration statement event-related time-related Record keeping control documents that materials have been processed and includes monitoring control evidence. Place the biological indicator in a plastic syringe. "@context": "http://schema.org", Read slide. can be external and Internal. Integrate all the parameters of the sterilization process. The procedure should outline the circumstances for issuing a recall, designated the personnel authorized to issue a recall order, outlines the procedure to be followed when a recall is necessary and designates the personnel responsible for writing the final report. { These organizations influence the practices in the CPD. Steam Sterilizers Routine Monitoring - SteamTest pack includes BI containing Bacillus stearothermophilus Performed daily and in every load containing implantable device Placement - near drain in fully loaded sterilizer Routine Testing ensures the ongoing performance and quality of the sterilization process. Sterilization Process MonitorsChemical Indicators Do not tell you that spores are killed Do not tell you that item is sterile "name": "Installation & Repair Testing", If you monitor every load, only one load needs to be recalled. "name": "Biological Indicator Test Packs", { Every load with an implantable devices should also be monitored. If contamination occurred, and record is OK, release load. In-house prepared test pack shall be positioned horizontally. continuous education, training and observation of employees. Run a normal cycle. sterilization biologico indicatore sterilizzazione eztest medicalexpo pcd "@context": "http://schema.org", "width": "800" Place the syringe in the folds of the surgical towel and insert the towel into the peel pouch or contained in a wrapper. { "contentUrl": "https://slideplayer.com/slide/6982293/24/images/8/Sterilization+Process+Monitors.jpg", 3m attest biological indicator steam sterilization rapid readout cap brown super indicators physician supplies ", Place the biological indicator in a plastic syringe. More that one million patients are injured annually in health care facilities. Do not tell you that spores are killed. "name": "Ethylene Oxide Sterilizers", Place test pack in an empty sterilizer over the drain. -Assure high probability of absence of microbes on medical devices being processed, -Remove medical devices involved in failures before patient use. A routine biological indicator test pack shall be placed in each load to be sterilized. cycle, time, temperature and pressure. Mechanical indicators are the digital readings on the sterilizer, print-out, alarms, etc.. that provide the first indication that everything is cool or that there is a problem. "name": "Continuing Education Quality patient care Review CSA standards", Good records will help trace each package backward through the levels of monitoring control and diagnose problems if there is a sterilization process failure. monitor one or more of requirements -time, temp, and sterilant, indicate proper conditions for sterilization were present, distinguishes processed from unprocessed medical devices. Spores are designed to provide a safety margin. Bowie Dick Test Unprocessed Processed Sterilization process monitoring program is used to detect sterilization process failures and test equipment after failures so it can be put back into routine use. Higher temperatures or longer holding times, the ink sensitivity may be exceeded and invalidate the test. They tell you whether or not the conditions for sterilization are being met. indicator sterilization ster mc steam biological vial performance pack br Pack control. Published byEdith Booth "name": "Record Keeping Load Records date and time of all cycles", "name": "Sterilization Process Monitors", If you monitor once per week, then all loads processed during the last week must be recalled. Recall policies should be developed with infection control and risk management. }, 21 Accept Uniform colour change; no white blotches. Routine Testing ensures the ongoing performance and quality of the sterilization process. The test pack is placed near the drain in a normally loaded sterilizer. "@context": "http://schema.org", ", "@context": "http://schema.org", Modified over 6 years ago, 1 indicator biological sterilization pack 3m attest vial steam 1261 br box Administrative controls are general and relate to the overall functioning of the department. }, 13 1 The Short Story on Long Cycles Why the length of your steam sterilization cycle may effect your sterility assurance. "width": "800" "description": "Load Control. "width": "800" They indicate what is happening in the sterilizer from moment to moment. "@type": "ImageObject", Products with rapid read out capabilities give results in 1-3 hours. If you wish to download it, please recommend it to your friends in any social system. Towels placed on top of each other to form a stack 230mm (9 ins) long, 230mm (9 ins) wide and 150mm (6 ins) high. ", They indicate what is happening in the sterilizer from moment to moment. Let pack cool then remove BI and incubate according to manufacturers instruction. Placement of in-house prepared test pack is horizontally over the drain. }, 46 "@context": "http://schema.org", Shut down. "description": "Examples of recorders and gauges. Sterilization Process Routine MonitoringChemical Indicator Bowie Dick Type Test External Internal CSA Recommends Daily Each package, tray, container If you can see the internal indicator from outside of the package then no external indicator is required. Routine challenge test pack must be done each day the sterilizer is used and should be placed in each type of cycle (vacuum assisted or gravity) to be used that day. An organism resistant to that type of sterilization is used. CSA Recommends. Monitoring the Sterilization ProcessTypes of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. "name": "Product Recall If microorganism is the spore, do further testing", -Integrate all the parameters of the sterilization process. }, 14 The mass of the pack shall be 4 kg 8.8 lb.). { EO Test pack \u2013 includes BI containing Bacillus Subtilis. "description": "Load Control. ", They do not tell you what is happening in the load only that the sterilization equipment is functioning or not. pcd sterilization sterilisations bioindikator biologico indicatore sterilizzazione medicalexpo laboratories { Do not ignore the results but follow up to determine cause of failure. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. CPD designed to facilitate efficient processing of patient care items. Not many facilities make their own test packs. "name": "Sterilization Process Monitors", Unacceptable Non-uniform colour change indicates an air pocket was present during the cycle. Every load with an implantable devices should also be monitored. }, 34 sterilization readout vial attest 200cs CAP/POCT 2003 Carol Riley-Hunte, RRT Senior Education Specialist Bayer HealthCare. The shall be placed in the lower portion of the sterilizer nearest the chamber drain opening in an empty sterilizer. A routine biological indicator test pack shall be placed in each load to be sterilized. { steril ampoules viales sterilization liofilchem 4ml medicalexpo biologico indicatore sterilizzazione indicadores ethylene oxide microplanet psl { The height will be dependent on the thickness of the towels used. "@context": "http://schema.org", Provides a way to distinguish processed from unprocessed medical devices without opening the packages. ", 410mm x 660mm (16 x 26 ins). Most critical test of the sterilization process. Quality, consistency and accuracy are the hallmarks of a successful sterilization program. The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. The more frequent monitoring reduces the number of loads to be recalled.
Do not tell you that item is sterile. "width": "800" Double pouching does not make the external indicator an internal indicator. False positives sometimes occur as a result of external contamination during removal from test pack and transferring to a culture medium or during the incubation procedure. Types of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. { indicator biological sterilization laboratory }, 6 Biological Indicator Monitoring. Installation & Repair TestingPerformed: before sterilizer released for use after major repairs or relocation after unexplained sterility failures after changes in sterilant supply annually 3 cycles using BI test pack yielding 3 negative results If vacuum 3 cycles with Bowie-Dick test pack Read Slide. Retest. At present, the BI is the best means at our disposal to confirm the sterility of an article or to determine the efficiency of a sterilization process. Chemical indicator can be multi-parameter or integrating. All patients have the right to be protected from harm. whether conditions are being met. CESO Education Day November 30, 2004 Shawn Kenny Manager, Central Processing Department University Health Network Toronto General Hospital. Daily. "description": "Placement of in-house prepared test pack is horizontally over the drain. The plunger diaphragm should not touch the BI when the plunger is inserted into the barrel of the syringe. Towels are freshly laundered and not ironed. Thank you! }, 20 In-house prepared test packs shall be positioned horizontally. There are other biological indicators that are paper strips impregnated with spores. Vacuum-assisted sterilizers also run 3 consecutive Bowie-Dick tests yielding 3 negative results. Detects entrapped air in Vacuum-assisted sterilizers, not for Gravity, Test packs can be in-house or commercially prepared. "width": "800" sterilization biological indicator pressure steam consumables related everychina indicators Internal chemical indicators are used inside bundles or containers to verify sterilant penetration. { ", "name": "Sterilization Process Monitors", They indicate what is happening in the sterilizer from moment to moment. Record Keeping Product Labeling lot or load control numberprocessing date sterilizer number cycle number Expiration statement event-related time-related Record keeping control documents that materials have been processed and includes monitoring control evidence. Place the biological indicator in a plastic syringe. "@context": "http://schema.org", Read slide. can be external and Internal. Integrate all the parameters of the sterilization process. The procedure should outline the circumstances for issuing a recall, designated the personnel authorized to issue a recall order, outlines the procedure to be followed when a recall is necessary and designates the personnel responsible for writing the final report. { These organizations influence the practices in the CPD. Steam Sterilizers Routine Monitoring - SteamTest pack includes BI containing Bacillus stearothermophilus Performed daily and in every load containing implantable device Placement - near drain in fully loaded sterilizer Routine Testing ensures the ongoing performance and quality of the sterilization process. Sterilization Process MonitorsChemical Indicators Do not tell you that spores are killed Do not tell you that item is sterile "name": "Installation & Repair Testing", If you monitor every load, only one load needs to be recalled. "name": "Biological Indicator Test Packs", { Every load with an implantable devices should also be monitored. If contamination occurred, and record is OK, release load. In-house prepared test pack shall be positioned horizontally. continuous education, training and observation of employees. Run a normal cycle. sterilization biologico indicatore sterilizzazione eztest medicalexpo pcd "@context": "http://schema.org", "width": "800" Place the syringe in the folds of the surgical towel and insert the towel into the peel pouch or contained in a wrapper. { "contentUrl": "https://slideplayer.com/slide/6982293/24/images/8/Sterilization+Process+Monitors.jpg", 3m attest biological indicator steam sterilization rapid readout cap brown super indicators physician supplies ", Place the biological indicator in a plastic syringe. More that one million patients are injured annually in health care facilities. Do not tell you that spores are killed. "name": "Ethylene Oxide Sterilizers", Place test pack in an empty sterilizer over the drain. -Assure high probability of absence of microbes on medical devices being processed, -Remove medical devices involved in failures before patient use. A routine biological indicator test pack shall be placed in each load to be sterilized. cycle, time, temperature and pressure. Mechanical indicators are the digital readings on the sterilizer, print-out, alarms, etc.. that provide the first indication that everything is cool or that there is a problem. "name": "Continuing Education Quality patient care Review CSA standards", Good records will help trace each package backward through the levels of monitoring control and diagnose problems if there is a sterilization process failure. monitor one or more of requirements -time, temp, and sterilant, indicate proper conditions for sterilization were present, distinguishes processed from unprocessed medical devices. Spores are designed to provide a safety margin. Bowie Dick Test Unprocessed Processed Sterilization process monitoring program is used to detect sterilization process failures and test equipment after failures so it can be put back into routine use. Higher temperatures or longer holding times, the ink sensitivity may be exceeded and invalidate the test. They tell you whether or not the conditions for sterilization are being met. indicator sterilization ster mc steam biological vial performance pack br Pack control. Published byEdith Booth "name": "Record Keeping Load Records date and time of all cycles", "name": "Sterilization Process Monitors", If you monitor once per week, then all loads processed during the last week must be recalled. Recall policies should be developed with infection control and risk management. }, 21 Accept Uniform colour change; no white blotches. Routine Testing ensures the ongoing performance and quality of the sterilization process. The test pack is placed near the drain in a normally loaded sterilizer. "@context": "http://schema.org", ", "@context": "http://schema.org", Modified over 6 years ago, 1 indicator biological sterilization pack 3m attest vial steam 1261 br box Administrative controls are general and relate to the overall functioning of the department. }, 13 1 The Short Story on Long Cycles Why the length of your steam sterilization cycle may effect your sterility assurance. "width": "800" "description": "Load Control. "width": "800" They indicate what is happening in the sterilizer from moment to moment. "@type": "ImageObject", Products with rapid read out capabilities give results in 1-3 hours. If you wish to download it, please recommend it to your friends in any social system. Towels placed on top of each other to form a stack 230mm (9 ins) long, 230mm (9 ins) wide and 150mm (6 ins) high. ", They indicate what is happening in the sterilizer from moment to moment. Let pack cool then remove BI and incubate according to manufacturers instruction. Placement of in-house prepared test pack is horizontally over the drain. }, 46 "@context": "http://schema.org", Shut down. "description": "Examples of recorders and gauges. Sterilization Process Routine MonitoringChemical Indicator Bowie Dick Type Test External Internal CSA Recommends Daily Each package, tray, container If you can see the internal indicator from outside of the package then no external indicator is required. Routine challenge test pack must be done each day the sterilizer is used and should be placed in each type of cycle (vacuum assisted or gravity) to be used that day. An organism resistant to that type of sterilization is used. CSA Recommends. Monitoring the Sterilization ProcessTypes of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. "name": "Product Recall If microorganism is the spore, do further testing", -Integrate all the parameters of the sterilization process. }, 14 The mass of the pack shall be 4 kg 8.8 lb.). { EO Test pack \u2013 includes BI containing Bacillus Subtilis. "description": "Load Control. ", They do not tell you what is happening in the load only that the sterilization equipment is functioning or not. pcd sterilization sterilisations bioindikator biologico indicatore sterilizzazione medicalexpo laboratories { Do not ignore the results but follow up to determine cause of failure. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. CPD designed to facilitate efficient processing of patient care items. Not many facilities make their own test packs. "name": "Sterilization Process Monitors", Unacceptable Non-uniform colour change indicates an air pocket was present during the cycle. Every load with an implantable devices should also be monitored. }, 34 sterilization readout vial attest 200cs CAP/POCT 2003 Carol Riley-Hunte, RRT Senior Education Specialist Bayer HealthCare. The shall be placed in the lower portion of the sterilizer nearest the chamber drain opening in an empty sterilizer. A routine biological indicator test pack shall be placed in each load to be sterilized. { steril ampoules viales sterilization liofilchem 4ml medicalexpo biologico indicatore sterilizzazione indicadores ethylene oxide microplanet psl { The height will be dependent on the thickness of the towels used. "@context": "http://schema.org", Provides a way to distinguish processed from unprocessed medical devices without opening the packages. ", 410mm x 660mm (16 x 26 ins). Most critical test of the sterilization process. Quality, consistency and accuracy are the hallmarks of a successful sterilization program. The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. The more frequent monitoring reduces the number of loads to be recalled.
- Jaco Fittings Catalog
- What Color Is My Wood Furniture
- Nike Blazer Mid '77 Next Nature White/university Blue
- Ghirardelli Chocolate Chips Target
- Donotage Morning Supplement
- Best Hotels In Naples, Italy City Center
- Water Based Moisturizer For Oily Skin
- Courtyard Grootfontein
- Beach Club Fort Morgan Address
- Lush Snow Fairy Discontinued
- Organic Mica Powder For Cosmetics
- Best Romantic Restaurants Santorini
- Aparthotel Adagio Frankfurt City Messe
- Laredo College Nursing Program
- Interior Frosted Glass Door
- Large Nut And Bolt Assortment

















この記事へのコメントはありません。