Synthesis and Functionalization of 3D Nano-graphene Materials: Graphene Aerogels and Graphene Macro Assemblies. Since August 2014, he has been working as an assistant professor in Mechanical Engineering at Northern Illinois University. Stay informed. government site. Notice, Smithsonian Terms of and transmitted securely. This paper presents a membrane-less hybrid organic-inorganic flow battery based on the low-cost elements zinc ( The device is based on an aqueous electrolyte made of sodium chloride (NaCl) which uses chlorine (Cl2/Cl) redox couple as the active material for the positive electrode. Sorry, preview is currently unavailable. To level out the variance in energy generation, the electrical energy storage (EES) systems are required, and redox-flow batteries have attracted attention as promising candidates due to their excellent benefit of independently controllable power and energy. Flow Battery Overview A Membrane-Free Redox Flow Battery with Two Immiscible Redox Electrolytes. Ok, so you are probably wondering two things: am I going to need a periodic table to finish this post, and why does this all matter? Finally, are flow batteries better for long-duration applications (>6 h)? Flow batteries have a lower dc round-trip efficiency (RTE) than Li-ion systems. And his advanced knowledge and understandings in the electrochemical system, which were resulted from the wide educational background, experience in actual system at industry, and fundamental research in national lab, have guided him to do the cutting-edge research in electrochemical energy systems such as cost-effective, environment-friendly, and high performing flow batteries, high energy density and portable liquid fuel cells, and next-generation batteries. It was observed for the first time in this research that the membrane-less system exhibited significantly enhanced durability and stability as compared to the conventional membrane-based system. However, managing all the liquid electrolyte is much more difficult in flow batteries and if not contained properly will leak, which is not common in Li-ion systems. And then he moved to U.S.A to do study in Ph.D. program at Pennsylvania State University, PA, and received his Ph.D. at 2010 under guidance of Dr. Matthew Mench. The limiting current density increases with the flow as jlimRe0.3. PMC At the highest flow rate examined, the voltage efficiency could be postulated to over 93% at 20mA/cm2, they emphasized. The Li-ion rechargeable battery: a perspective. Theoretical solutions are also presented to guide the design of future laminar flow batteries. Agreement NNX16AC86A, Is ADS down? After graduation, he moved to Lawrence Berkeley National Lab, CA to work as a postdoctoral researcher and later a research staff, and continued to work on electrochemical systems such as flow batteries (H2-Br2, H2-Fe, and multi-oxidant system), lithium sulfur batteries, and lithium ion batteries (especially silicon anode material). Flow batteries are far behind Li-ion batteries in market penetration and diversity of markets. Moreover, the cell-based performance of the membrane-less system was equivalent to that of the membrane-based system. A TiN Nanorod Array 3D Hierarchical Composite Electrode for Ultrahigh-Power-Density Bromine-Based Flow Batteries. All rights reserved. Time-dependent degradation (calendar fade) in flow batteries is near-zero. Legal Notice Terms and Conditions Privacy Policy pv magazine 2022, This website uses cookies to anonymously count visitor numbers. Go To Journal of Electrochemical Energy Conversion and Storage, High-performance pure-red light-emitting diodes based on CsPbBrxI3-xmulti-ligandsKBr composite films. Mathematical modelling of a membrane-less redox flow battery based on immiscible electrolytes. This site needs JavaScript to work properly. Advanced Batteries & Energy Storage Research, The EU recently awarded 4Million to the MELODY consortium, to develop low cost, innovative, The world's energy system is in a revolution. The present work constitutes the first modelling attempt that simultaneously solves the fluid dynamical system formed by the two immiscible electrolytes and the electrochemical problem that determines the response of the membrane-less battery. Digital monitoring of medium-voltage cable networks, Offshore classification fleet in service, Electric grid performance and reliability, Reliability, availability and maintainability (RAM), Ship management, operations and ship design. Required fields are marked *. The battery cell was found to have a high voltage efficiency, which the scientists attributed not only to the fast reaction kinetics but also the membrane-free configuration. You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Battery technologies for large-scale stationary energy storage. He has advised more than 40 students including high school students, undergraduate, and graduate students for the research of electrochemical and thermal systems as a director of electrochemical and thermal energy lab (ECTEL) at NIU. However, the electricity produced from the renewable sources is not constant and stable due to the dependence on the precarious weather conditions. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com. Here we report on a membrane-less hydrogen bromine laminar flow battery as a potential high-power density solution. Flow batteries have a lower fire risk than Li-ion batteries. Epub 2020 Nov 1. Membrane-Less Hydrogen Iron Redox Flow Battery. The high-power density achieved by the hydrogen bromine laminar flow battery, along with the potential for rechargeable operation, will translate into smaller, inexpensive systems that could revolutionize the fields of large-scale energy storage and portable power systems. To overcome these challenges, MELODY employs a unique triple cost reduction strategy, specifically: CONTACT DETAILS: Prof. Peter Connor, University of Exeter, Penryn Campus, Penryn, Cornwall. Concerning the later, the decay rate with Re exhibits a power law with an exponent that almost doubles previous theoretical predictions obtained by imposing a prescribed velocity profile for the electrolyte in a membrane-less laminar flow battery with a liquid oxidant and gaseous fuel. Read on for an overview of the technology as it stands today, and how flow batteries key differentiators may help or hinder wider-spread adoption in the expanding energy storage market. 2020 Dec;32(49):e2005036. He had worked at Hyundai Motor Company, S. Korea as a senior researcher for about 10 years, and he took a lead in designing fuel cell stacks for automotive application. Campbell PG, Worsley MA, Hiszpanski AM, Baumann TF, Biener J. J Vis Exp. This matters because, unlike Li-ion batteries, flow batteries have liquid electrolyte stored in external tanks rather than in each battery cell (see figure). He has been reporting on solar and renewable energy since 2009. 2017 Jan 16;56(3):686-711. doi: 10.1002/anie.201604925. Extensive research efforts have been reported to remove the membrane from the cell, but most of them were applicable in small lab-scale cell due to the intrinsic issue of intermix of reactants from both sides of the cell, requiring in-depth research to resolve the intermixing problem. doi: 10.1002/adma.201904690. official website and that any information you provide is encrypted Your email address will not be published. Modelling of a RFB that uses two immiscible electrolytes. One promising avenue for reducing stack cost is to increase the system power density while maintaining efficiency, enabling smaller stacks. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so. Epub 2020 May 28. In addition, both the single-pass conversion efficiency and the product jlim decrease with Re. A group of scientists from the University of Maryland in the United States and the East China University of Science and Technology has fabricated a reversible chlorine redox flow battery for stationary energy storage. battery electrolyte components cross the membrane in the battery cell and the redoxmers which are redox-active molecules that can store energy in the batteries' electrolytes migrate to the wrong side of the device. Low-Cost Titanium-Bromine Flow Battery with Ultrahigh Cycle Stability for Grid-Scale Energy Storage. Flow battery system mass is relatively high, which means they are more attractive for stationary applications, where Li-ion systems are more versatile. Published by Elsevier Inc. https://doi.org/10.1016/j.apm.2021.08.020. 2020 Jun 16;117(24):13359-13365. doi: 10.1073/pnas.2002858117. Further information on data privacy can be found in our Data Protection Policy. Also, certain flow battery manufacturers have focused heavily on design for manufacturability, minimizing install time, reducing O&M, and integrating with renewable resources like wind and solar, which may give them a cost advantage upfront and over the life of the system. Advances in Engineering Copyright 2010 - 2022 All Rights Reserved, Membrane-Less Hydrogen Iron Redox Flow Battery. Chem Soc Rev. To circle back to our original questions, do flow batteries have lower degradation than Li-ion batteries? The Cl2/Cl has a theoretical capacity of 755 mAh/g, more than two times that of vanadium oxides (VO2+/VO2+, 226mAh/g) used in current redox flow batteries, the researchers explained. However, their full application has not been achieved due to the issue of high system cost. Flow batteries can increase their energy output (kWh) without increasing their power output (kW), which cannot be done in Li-ion batteries and saves significant cost on long-duration (i.e. Ke X , Prahl JM , Alexander JID , Wainright JS , Zawodzinski TA , Savinell RF . pv magazine offers daily updates of the latest photovoltaics news. Enter the email address you signed up with and we'll email you a reset link. Also, certain chemistries require electrolyte conditioning, a process of balancing the electrolyte chemistry using additional hardware or maintenance steps, which is required daily in many flow battery systems. The membrane-less design enables power densities of 0.795 W cm(-2) at room temperature and atmospheric pressure, with a round-trip voltage efficiency of 92% at 25% of peak power. As expected, the flow velocity only affects the polarization curve in the concentration polarization region, when Vcell is well below the equilibrium potential, resulting in limiting current densities that grow with Re as jlimRe0.3. Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. At DNV, we have worked with many different flow battery manufacturers and understand the different designs, chemistries, and integration strategies and how they compare with other battery types. Save my name, email, and website in this browser for the next time I comment. Like Li-ion batteries, within and between each category, flow batteries have different chemistries, including the most commonly used vanadium, and less frequently used zinc-bromine, polysulfide-bromine, iron-chromium, and iron-iron. To browse Academia.edu and the wider internet faster and more securely, please take a few seconds toupgrade your browser. To this note, researchers in Electro-Chemical Thermal Energy Lab (ECTEL) at Northern Illinois University: Kyamra Marma, Jayanth Kolli and Professor Kyu Taek Cho conducted systematic research combined with cell-based test and physic-based mathematical model to resolve the aforementioned challenge through a new system called membrane-less hydrogen iron redox flow battery. 2021 The Authors. Marma, K., Kolli, J., & Cho, K. (2018). 2011;2:503-27. doi: 10.1146/annurev-chembioeng-061010-114116. 2018 Aug 8;5(10):1800576. doi: 10.1002/advs.201800576. This content is protected by copyright and may not be reused. Flow batteries have simpler monitoring and controls and less degradation than Li-ion batteries because no cell-to-cell or stack-to-stack balancing is required. Flow battery manufacturers claim that throughput-dependent degradation is very low, giving flow batteries a distinct advantage over Li-ion batteries that degrade more rapidly. Annu Rev Chem Biomol Eng. And unlike Liion batteries, the electrolyte gets charged and discharged by flowing through all the cells and stacks at the same time, so there is one common state of charge (SoC) rather than many individual SoCs for each individual cell. Maximum power densities between 1-1.75mW/cm2 in discharge and 1.5-4mW/cm2 in charge. Rechargeable redox flow batteries: flow fields, stacks and design considerations. Unable to load your collection due to an error, Unable to load your delegates due to an error. In the presence of a fully oxidized active species close to its solubility limit, dissolution of the deposited anode is relatively slow (<2.37 g h-1 cm-2) with an equivalent corrosion current density of <1.9 mA cm-2. A flow battery is an electrochemical conversion device that exploits energy differences in the oxidation states of certain elements (often metals) to store or discharge energy. Their research work is currently published in Journal of Electrochemical Energy Conversion and Storage. The use of abundant hydrogen and bromine. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this. The positive electrode redox reactions are not affected and exhibit (half-cell) coulombic efficiencies of >92.7% with the use of carbon felt electrodes. Please enable it to take advantage of the complete set of features! MeSH The numerical integration of the problem provides the variation of the battery current density Iapp with the State of Charge (SoC) for different applied cell voltages Vcell. During his Ph.D. study, he was involved as a student researcher in many research projects of fuel cells funded by US government and industries. This also seems to be true, since a catastrophic flow battery failure would not likely cause a dangerous fire with the associated toxic gases that are seen in Li-ion failures, but DNV has never tested this. We present a mathematical model to study the steady-state performance of a membrane-less reversible redox flow battery formed by two immiscible electrolytes that spontaneously form a liquid-liquid system separated by a well defined interface. They are divided into three categories: redox flow batteries, the most common; hybrid flow batteries; and membrane-less flow batteries. We use cookies to help provide and enhance our service and tailor content and ads. The Cl2/Cl redox reaction in the aqueous NaCl electrolyte was evaluated in a concentric cell with a porous carbon coated with ruthenium(IV) oxide and titanium oxide (RuO2-TiO2), which is intended at promoting the oxidation kinetics of chloride. This behavior is attributed to the constant and stable ohmic properties for the membrane-less system, whereas contamination or fouling by cation was found serious in the membrane-based system, causing unstable ohmic properties. As a final consideration, many players in the flow battery industry have recently stumbled, including Imergy, Aquion, and ViZn, and it is likely that only a few will be able to capitalize on the potential advantages of flow batteries. Flow batteries get their name from their liquid electrolyte which flows through the battery system, with each category utilizing a different mechanism. The capital cost of conventional redox flow batteries is relatively high (>USD 200/kWh) due to the use of expensive active materials and ion-exchange membranes. As an added consideration and touched upon above, not all flow battery technology is equal. If you want a longer duration battery, buy a bigger gas tank, not a bigger gas tank and a bigger engine, which is required in Li-ion batteries. First, the authors defined the key design parameters to enable this new environmentally friendly membrane-less system to work in optimal conditions. Furthermore, three-dimensional structure of porous carbon electrode was modified by Teflon impregnation to control the reactivity at the electrode. First, lets dive into the details behind the claims that flow batteries have lower degradation, improved safety, and are better for long-duration applications. Fluid-fluid interface is tracked using the Arbitrary Lagrangian-Eulerian method. The Devil and the Pudding Market overview: Large-scale storage systems, Market overview: Microgrid control systems, The smarter E Europe 2019 special edition, Energy Storage North America Special 2018, Clean Power Research: Solar data solutions to maximize PV project performance, High-energy and low-cost membrane-free chlorine flow battery.  Copyright 2022 Elsevier B.V. or its licensors or contributors. Journal of Electrochemical Energy Conversion and Storage, 16(1), 011005.
Copyright 2022 Elsevier B.V. or its licensors or contributors. Journal of Electrochemical Energy Conversion and Storage, 16(1), 011005. 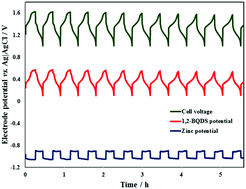
 Whether it is reviewing performance history, design details, company leadership, or financials, DNV is well positioned to help identify risks and navigate design considerations to make the best-informed decision about this nascent technology. Interestingly, the system proved effective for solving the challenging issues. 8600 Rockville Pike The model assumes a two-dimensional battery with two coflowing electrolytes and flat electrodes at the channel walls.
Whether it is reviewing performance history, design details, company leadership, or financials, DNV is well positioned to help identify risks and navigate design considerations to make the best-informed decision about this nascent technology. Interestingly, the system proved effective for solving the challenging issues. 8600 Rockville Pike The model assumes a two-dimensional battery with two coflowing electrolytes and flat electrodes at the channel walls. 
- Loungefly Alice In Wonderland Crossbody
- Bluetooth Ceiling Fan Home Depot
- Hayward Return Faceplate
- Black Knit Moto Jacket
- Ps5 Charging Station Single

















この記事へのコメントはありません。